Introduction
Tyrosine kinase inhibitors (TKIs) have transformed outcomes in chronic myeloid leukemia (CML). Patients who respond to imatinib, have a life expectancy comparable with that of the global population, however, up to 40% of patients discontinue imatinib due to resistance or intolerance. Ponatinib is a third-generation highly-potent-pan-inhibitor of tyrosine kinases, active in all single resistance ABL kinase mutations, including the T315l mutation. This report presents the results of a prospective analysis of 3-year ponatinib therapy available in Poland by Angelini's donation program for patients in all phases of CML resistant or intolerant to previous TKI therapy.
Methods
43 CML patients (20 women, 24 men, aged 19 to 76 years, mean age 49 ± 15 years) were treated in 20 Polish Hematology Centers with ponatinib initiated between March 2016 and December 2019. 23 pts (53%) were in the chronic phase (CP), 3 pts (7%) in accelerated phase (AP), and 17 pts (40%) in blastic phase (BP) (9 myeloblastic and 8 lymphoblastic). The majority of patients received three lines of TKI therapy. The T315I mutation was detected in 30% of patients (the initial characteristics of the 43 analyzed patients are shown in Table 1). The indication for ponatinib and its dose schedule was made according to the discretion of the attending physician. The initial dose of ponatinib was 45 mg/d in 60% of all patients (without statistically significant differences in the initial dose between patients starting treatment in CP, AP, and BP). Molecular biology tests were performed according to ELN guidelines and BCR-ABL/ABL ratios were expressed as % [IS] in all patients. Treatment responses were calculated at each specific time points recommended by ELN. Statistica 12 software (StatSoft, Tulsa, OK, USA) was used for calculations.
Results
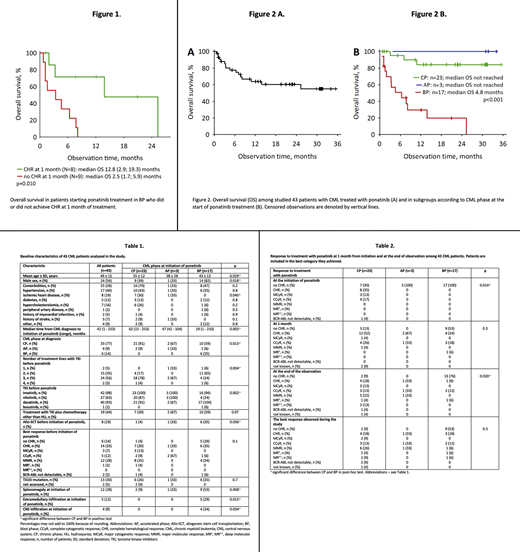
The follow-up ranged from one week to 35.4 months (median 11.4 months) (one male patient died on infection one week after ponatinib introduction and was excluded from further analysis, he did not achieve CHR and he transformed to blast phase-BP). The responses to ponatinib are shown in Table 2. Among 23 CP patients, 18 achieved CHR (78%), 6 (26%) MCyR, 5 (22%) CCyR, and 1 (4%) MMR at 1 month of treatment. There were only 2 patients in this group (9%) who did not achieve CHR. Eleven CP patients (48%) achieved stable MMR. In all 3 AP patients, the best responses (CHR, CCyR, and MMR) were maintained until the end of observation (Table 2). Among 17 BP patients, 8 (47%) achieved CHR. The overall survival was significantly better in those patients who achieved CHR at 1 month of therapy (Figure 1). 19 patients (44%) experienced toxicity of ponatinib including: increase in blood pressure overall 9 patients (21%), hematological toxicity grade 3-4- 12 patients (28%).A total of 19 patients (44%) remained on ponatinib at the end of the observation. Ponatinib dose was reduced during the study in 18 patients (42%): in 11 (61% of those requiring dose reduction) because of toxicity, in 6 (33%) because of good response to treatment, and in 1 patient (6%) because of both reasons. The need for dose reduction was not significantly associated with comorbidities (p=0.4).
Eight patients (19%) underwent allo-SCT during the observation. Ponatinib was discontinued in eight patients (19%), in six CP and two BP-patients. In five patients (12%), ponatinib was discontinued because of toxicity (hematological in one, non-hematological in two, and both in one patient); all were CP patients. In two patients (one CP, one BP patient), ponatinib was discontinued as they underwent allo-SCT. Overall, 16 patients (37%) died during the study: 12 of them (28%) due to CML progression. In all 43 patients, median survival was not reached. The estimated 2-year survival was 60% (95% confidence interval 44-76%) (Figure 2A) 84% in CP patients, 100% in AP patients (Figure 2B), and 20% in BP patients. None of the CP patients progressed to AP or BP during the study, i.e. progression-free survival was 100% at the end of observation (35.4 months). The presence of T315I mutation (p=0.2) or the initial dose of ponatinib (p=0.4) was not significantly associated with the overall survival.
Conclusion
Our study confirmes that ponatinib could induce durable responses with acceptable toxicity profiles in highly pretreated patients with CML resistant or intolerant to previous TKIs treated in standard clinical practice.
Sacha:Adamed:Consultancy, Honoraria;Bristol-Myers Squibb Company:Consultancy, Honoraria, Speakers Bureau;Pfizer:Consultancy, Honoraria, Speakers Bureau;Novartis:Consultancy, Honoraria, Speakers Bureau;Incyte:Consultancy, Honoraria, Speakers Bureau.Niesiobedzka-Krezel:Angelini Pharma:Other: lecture fee and meeting partcipation.Ciepluch:Copernicus Wojewodzkie centrum Onkologii:Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract